The ADR CP is the "all in one" representation of adverse drug risks which helps the user to keep the overview over kinetic and dynamic adverse drug reactions and thereby take the fast track to drug safety and avoidance of serious medication errors when prescribing. The core of the ADR CP consists of a highly innovative and dynamic literature based drug interaction checker which reconciles the control of MDDI (Multi-Drug Drug Interactions) and ADR (Adverse Drug Risks) in a patient medication. Thereby, the ADR CP makes the impact of kinetic and dynamic drug interactions on vital signs and adverse drug risks of major importance transparent and recognizable at one glance.
Please look at the ADR CP for (fictitious) patient Willy Nottingham in the screen below:
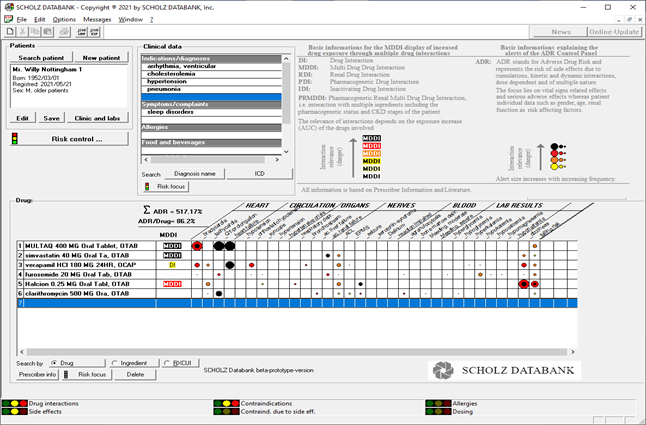
Please look at the ADR CP for (fictitious) patient Willy Nottingham in the screen below:

The new e²CP technology (“electronic express ChromaPictography”) provides the ADR CP even for complex medications within seconds. It reminds of running chromatograms in a chemist lab, however with very high speed and performance.
The MDDI technology supports thereby a completely new view on drug drug interactions. Thus, the user recognizes immediately, how one drug is kinetically affected by ALL other drugs of the medication. The MDDI alerts bring this message to the user; they symbolize the interaction magnitude by different colours from light yellow over red to black. The adverse drug risks focussed on are dependent on the magnitude of the interactions. They are in particular related to the vital signs and serious drug side effects as listed in the table above which is the center of the ADR CP. Size and color of the ADR alerts in the cells visualize the relevance and frequency of the adverse effects. The ADR CP develops its power especially in complex polypharmacy scenarios and when treating multimorbide elderly patients. Individual patient data such as renal failure stages or pharmacogenetics may be included in the risk analysis, whereby the ADR CP becomes an effective tool of personalized or precision medicine.
The ADR CP of SDB comes as MS Windows executable local network software; this software (SDB ADR CP) can be used stand-alone; SDB ADR CP can also be called through an interface from third party EMR software and includes the option to submit coded patient data such as drugcodes, ICD10-Codes and other patient individual information. Whereas this solution comes with a graphic UI the ADR CP can also be built as a webbased service with UI using the SDB webservice based on REST Technology.
The Beta-Version of the ADR CP is now available for doctors, pharmacists, and developers interested in next generation drug safety software to prevent medication errors. Be part of this exciting development and contact us to participate as ADR CP Beta-Tester!

