In 2020 SCHOLZ DataBank founder Wolfgang Scholz, through his german pharmacy house of origine „Hirsch-Apotheke“, had closed a contract with the Westphalian-Wilhelms-Universitiy Münster in Germany for the research of a COVID-19 drug.
This research had the target to focus on drugs, in particular of phytotherapeutic nature, which are already well known and proven in the treatment of other diseases, an endeavour known also under the name „repurposing“.
A screening of about 20 preparations, which had been discussed in the pharmaceutical research committee (Wolfgang Scholz, pharmacist and research funder, Prof. Dr. Georg Hempel, Clinical Pharmacy, and Prof. Dr. Andreas Hensel, Pharmaceutical Biology) and which seemed promising from the pharmaceutical point of view, was started and a potential anti-SARS-CoV-2 medication (ASM-1) could be identified in the virologic labs of the university, headed by Prof. Dr. Stephan Ludwig. Thereby, in a test system with usual cell cultures the toxicity and the efficacy of ASM-1 was explored.
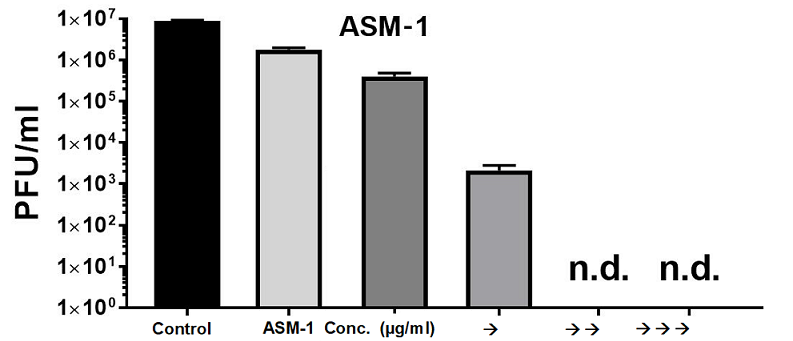
Based on the results it can be concluded that the concentrations of ASM-1 needed for the antiviral effect are substantially lower then the cytotoxic concentrations thereby opening an appropriate therapeutic window and index. The antiviral effect against the corona virus SARS-CoV-2 manifested on the one hand in tests with a so-called pseudovirus. This virus carries the SARS-CoV-2-Spike-Protein on its surface which is essential for the docking of the virus at the cell and the consecutive penetration into the cell, wheryby the cell gets finally sick and becomes the site of virus replication. ASM-1 showed a strong effect to inhibit the penetration of the virus into the cell in the pseudovirus assay. The antiviral effect was confirmed on the other hand in tests where cells were directly infected through the real corona virus, and this effect was even stronger compared to the pseudovirus assay. A concentration-dependent inactivation could be observed which was so strong that the virus was not detectable in the cells when higher doses of ASM-1 were applied. Process and results of the tests indicate that not only the penetration of the virus into the cell might be blocked but also the virus replication in the cell inhibited. These assumptions have to be elucidated more specifically by more lab assays.
ASM-1 belongs to a group of plant-based medications for which experiences in the treatment of other diseases exist and which show usually a good tolerability. Therefore best preconditions exist to start the clinical research with ASM-1 and to explore how this medication might contribute to the prophylaxis and/or therapy of COVID-19.
„We have reached in a relative short period of only a few months a very important mile stone“, said Wolfgang Scholz, pharmacist, and the initiator and funder of the research project.

„Now is the time, parallelly to the deepening of the experimental insights, to get the process of the clinical research started and to attract clinicians in order to obtain knowledge if ASM-1 can finally be successfully administered to COVID-19 patients and to partner with a highly competent company of the pharmaceutical industry. Insofar this message is an invitation to clinical research institutions as well as to the pharmaceutical industry to support the development and the deployment of ASM-1 and thereby to contribute as fast as possible to a successful fight against the pandemic.“
If you are interested in supporting the clinical research or if you have further questions concerning ASM-1 please write to [email protected] .