New drugs bring up all the time new drug interactions and adverse drug risks
which should be known and avoided, for example the new kinase inhibitor quizartinib, now under the brand name "Vanflyta" in the market.
which should be known and avoided, for example the new kinase inhibitor quizartinib, now under the brand name "Vanflyta" in the market.
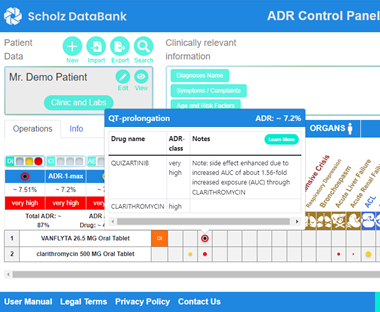
SCHOLZ Databank with the ADR Control Panel is the powerful instrument to be up-to-date and stay in control, when a patient in need of quizartinib is already on or needs also other drugs. Quizartinib causes very frequently neutropenia and frequently bone marrow depression; therefore bacterial or fungal infections may occur which require treatment with antibiotics or antimycotics. Then the question may come up which might be the preferred antimycotic, for example ketoconazole or fluconazole. Or which antibiotic might be more appropriate to avoid an increase in the major risk of quizartinib which is a frequent prolongation of the QT interval and occasional dangerous Torsades de pointes.
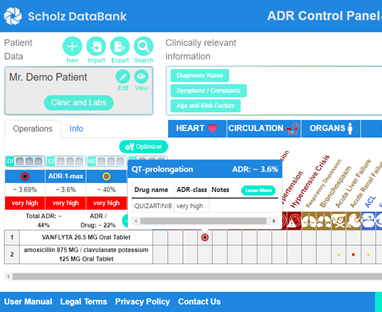
The ADR Control Panel of SCHOLZ Databank supports the user to compare diverse alternatives and provides quickly answers, for example, that both clarithromycin and azithromycin cause a substantial additional risk of dangerous arrhythmias compared to beta-lactam antibiotics such as cefaclor or amoxicillin with or without clavulanic acid. In particular, on top of the QT prolonging properties of the macrolides, the major problem of clarithromycin as a strong CYP3A4 blocker becomes visible leading to an unwanted inhibition of the quizartinib metabolism and a substantially higher plasma level of the kinase inhibitor enhancing adverse effects.
Stay up-to-date when e-prescribing!
The ADR CP is your fast track to control Drug Risks and to more Drug Safety supporting doctors with
● a highly innovative and dynamic drug drug interaction checker
● the "all in one" overview of drug risks in complex patient medications
● reconciliation of multiple kinetic interactions and adverse drug effects
● an Optimizer to find safer drug alternatives.
If you want more information about the ADR CP then click here.
The ADR Control Panel of SCHOLZ Databank supports the user to compare diverse alternatives and provides quickly answers, for example, that both clarithromycin and azithromycin cause a substantial additional risk of dangerous arrhythmias compared to beta-lactam antibiotics such as cefaclor or amoxicillin with or without clavulanic acid. In particular, on top of the QT prolonging properties of the macrolides, the major problem of clarithromycin as a strong CYP3A4 blocker becomes visible leading to an unwanted inhibition of the quizartinib metabolism and a substantially higher plasma level of the kinase inhibitor enhancing adverse effects.


Stay up-to-date when e-prescribing!
The ADR CP is your fast track to control Drug Risks and to more Drug Safety supporting doctors with
● a highly innovative and dynamic drug drug interaction checker
● the "all in one" overview of drug risks in complex patient medications
● reconciliation of multiple kinetic interactions and adverse drug effects
● an Optimizer to find safer drug alternatives.
If you want more information about the ADR CP then click here.

